Results
![]() Simulations were performed for TCRs of length N=5, with amino-acid compositions typical of human proteome.
Simulations were performed for TCRs of length N=5, with amino-acid compositions typical of human proteome.
Selected fraction:
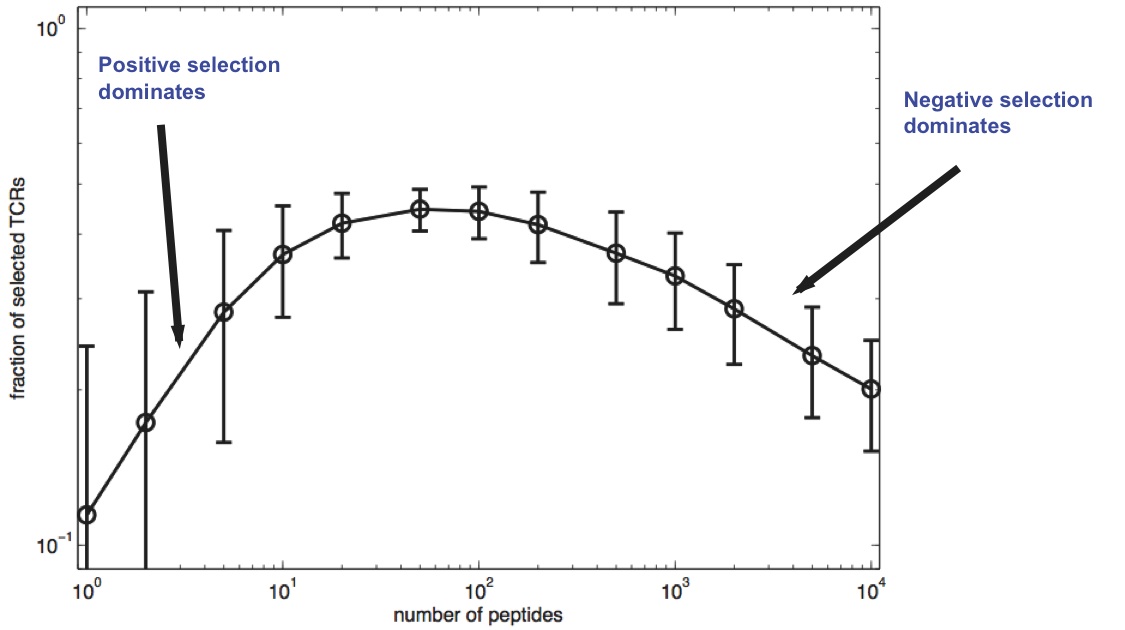
(Typical number of peptide encounters in thymus is 1,000-10,000)
The selection process biases the composition of amino-acids in mature TCRs:
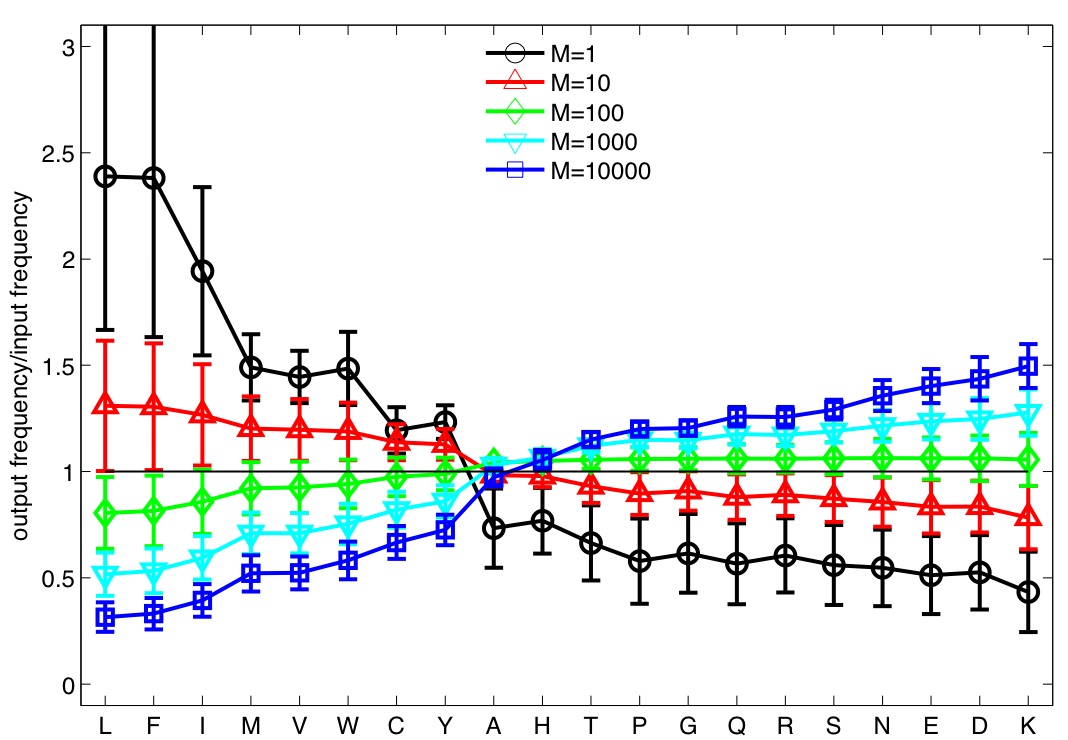
Negative selection leads to a preference for weaker amino-acids.
Is this consistent with TCR amino-acid compositions?
In 2008, we examined limited available crystallographic data from TCR/peptide interfaces:
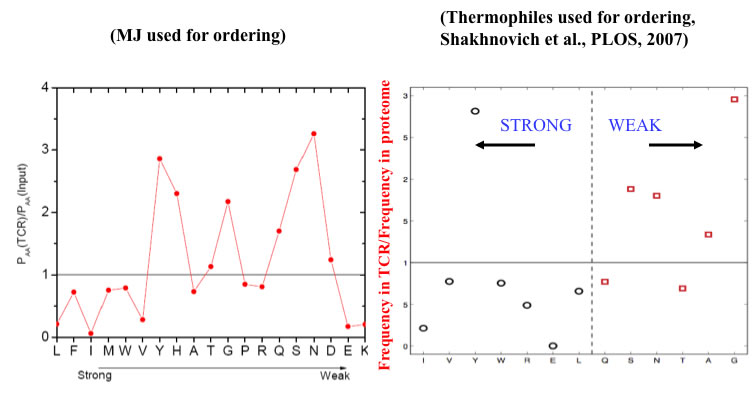
A more recent analysis is less clear, and more work needs to be done:
Y. Elhanati, A. Murugan, C.G. Callan,T. Mora, & A.M. Walczak, PNAS 111, 9875 (2013) (offlline)
Any preferences could also reflect (evolutionary selected) bias in generation of candidate TCRs.
![]() Specificity of TCRs and transgenic mice:
Specificity of TCRs and transgenic mice: 
E.S. Huseby, F. Crawford, J. White, P Marrack & J.W. Kappler, Nat Immunol 7, 1191 (2006)
Created transgenic mice, expressing only one self-peptide (M=1) in their thymus!
Compared specificity of TCRs from normal and transgenic mice to mutantations of a recognized peptide:
Normal mice TCR typically do not recognize peptides after single site mutations, transgenic TCR do.
In normal mice TCR/peptide binding is due to many weak bonds, in transgenic mice a few strong bonds suffice.
![]() Elite controllers of HIV appear to better tolerate mutations of the virus. Is a similar mechanism at play?
Elite controllers of HIV appear to better tolerate mutations of the virus. Is a similar mechanism at play?
A. Košmrlj, ..., A.K. Chakraborty, Nature online (5 May 2010) WebMed, ...
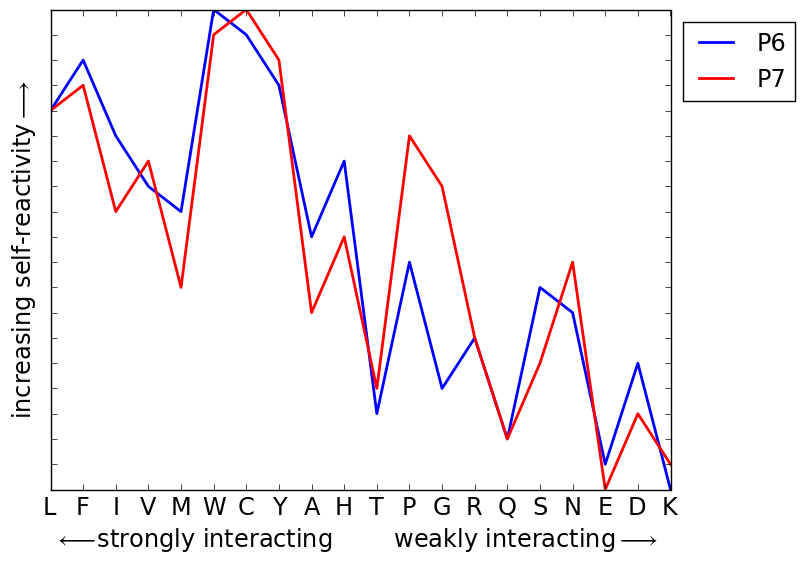
![]() Self-reactive TCR (implicated in autoimmune disease) are enriched in stronger binding amino-acids:
Self-reactive TCR (implicated in autoimmune disease) are enriched in stronger binding amino-acids:
B.D. Stadinski, ..., A.K. Chakraborty & E. Huseby, Nature Immunology (2006)